Bromfenac Ophthlamic Solution Prescribing Information
Package insert / product label
Generic name: bromfenac sodium
Dosage form: ophthalmic solution
Drug class: Ophthalmic anti-inflammatory agents
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Use In Specific Populations
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
BROMFENAC ophthalmic solution, 0.075%, for topical ophthalmic use
Initial U.S. Approval: 1997
Indications and Usage for Bromfenac Ophthlamic Solution
Bromfenac ophthalmic solution is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery. (1)
Bromfenac Ophthlamic Solution Dosage and Administration
Instill one drop of bromfenac ophthalmic solution to the affected eye twice daily (morning and evening) beginning 1 day prior to surgery, the day of surgery, and 14 days postsurgery. (2.1)
Dosage Forms and Strengths
Topical ophthalmic solution: bromfenac, 0.075%. (3)
Contraindications
None (4)
Warnings and Precautions
Adverse Reactions/Side Effects
The most commonly reported adverse reactions in 1 to 8% of patients were: anterior chamber inflammation, headache, vitreous floaters, iritis, eye pain and ocular hypertension. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2024
Full Prescribing Information
1. Indications and Usage for Bromfenac Ophthlamic Solution
Bromfenac ophthalmic solution, 0.075% is indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery.
2. Bromfenac Ophthlamic Solution Dosage and Administration
2.1 Recommended Dosing
One drop of bromfenac ophthalmic solution should be applied to the affected eye twice daily (morning and evening) 1 day prior to surgery, the day of surgery, and 14 days postsurgery.
2.2 Use with Other Topical Ophthalmic Medications
Bromfenac ophthalmic solution, 0.075% should be administered at least 5 minutes after instillation of other topical medications. Bromfenac ophthalmic solution, 0.075% may be administered in conjunction with other topical ophthalmic medications such as alpha-agonists, beta-blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics.
5. Warnings and Precautions
5.1 Slow or Delayed Healing
All topical nonsteroidal anti-inflammatory drugs (NSAIDs), including bromfenac ophthalmic solution, 0.075%, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.2 Potential for Cross-Sensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs, including bromfenac ophthalmic solution, 0.075%. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.3 Increased Bleeding Time of Ocular Tissue
With some NSAIDs, including bromfenac ophthalmic solution, 0.075%, there exists the potential for increased bleeding time due to interference with platelet aggregation. There have been reports that ocularly applied NSAIDs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that bromfenac ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
5.4 Keratitis and Corneal Reactions
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs, including bromfenac ophthalmic solution, 0.075%, and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days postsurgery may increase patient risk for the occurrence and severity of corneal adverse events.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Slow or Delayed Healing[see Warnings and Precautions (5.1)]
- Potential for Cross-Sensitivity [see Warnings and Precautions (5.2)]
- Increased Bleeding Time of Ocular Tissue [see Warnings and Precautions (5.3)]
- Keratitis and Corneal Reactions [see Warnings and Precautions (5.4)]
- Contact Lens Wear [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions in 1 to 8% of patients were: anterior chamber inflammation, headache, vitreous floaters, iritis, eye pain and ocular hypertension.
8. Use In Specific Populations
8.1 Pregnancy
There are no adequate and well-controlled studies in pregnant women to inform any drug associated risks. Treatment of pregnant rats and rabbits with oral bromfenac did not produce teratogenic effects at clinically relevant doses.
Clinical Considerations
Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetal cardiovascular system (closure of ductus arteriosus), the use of bromfenac ophthalmic solution during late pregnancy should be avoided.
Data
Animal Data
Treatment of rats with bromfenac at oral doses up to 0.9 mg/kg/day (195 times a unilateral daily human ophthalmic dose on a mg/m2 basis, assuming 100% absorbed) and rabbits at oral doses up to 7.5 mg/kg/day (3243 times a unilateral daily dose on a mg/m2 basis) produced no structural teratogenicity in reproduction studies. However, embryo-fetal lethality, neonatal mortality and reduced postnatal growth were produced in rats at 0.9 mg/kg/day, and embryo-fetal lethality was produced in rabbits at 7.5 mg/kg/day.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.2 Lactation
There are no data on the presence of bromfenac in human milk, the effects on the breastfed infant, or the effects on milk production; however, systemic exposure to bromfenac from ocular administration is low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for bromfenac and any potential adverse effects on the breast-fed child from bromfenac or from the underlying maternal condition.
11. Bromfenac Ophthlamic Solution Description
Bromfenac ophthalmic solution, 0.075% is a sterile aqueous, topical NSAID, for ophthalmic use. The USAN name for bromfenac sodium sesquihydrate is bromfenac sodium. Bromfenac sodium is designated chemically as sodium [2-amino-3-(4-bromobenzoyl) phenyl] acetate sesquihydrate, with an empirical formula of C15H11BrNNaO3• 1½H2O. The structural formula for bromfenac sodium sesquihydrate is:
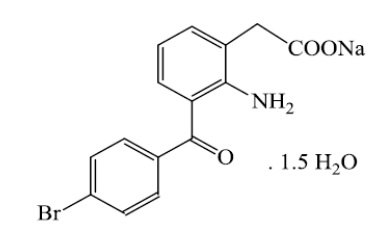
Bromfenac sodium is a bright orange to yellow powder. The molecular weight of bromfenac sodium sesquihydrate is 383.17. Bromfenac ophthalmic solution is a greenish-yellow to dark yellow viscous liquid with an osmolality between 265 and 335 mOsmol per kg.
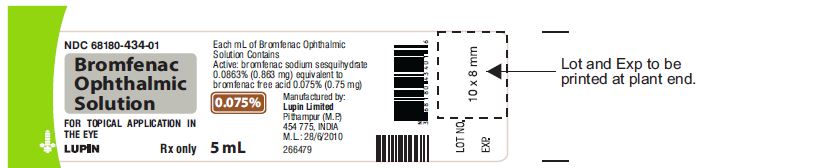
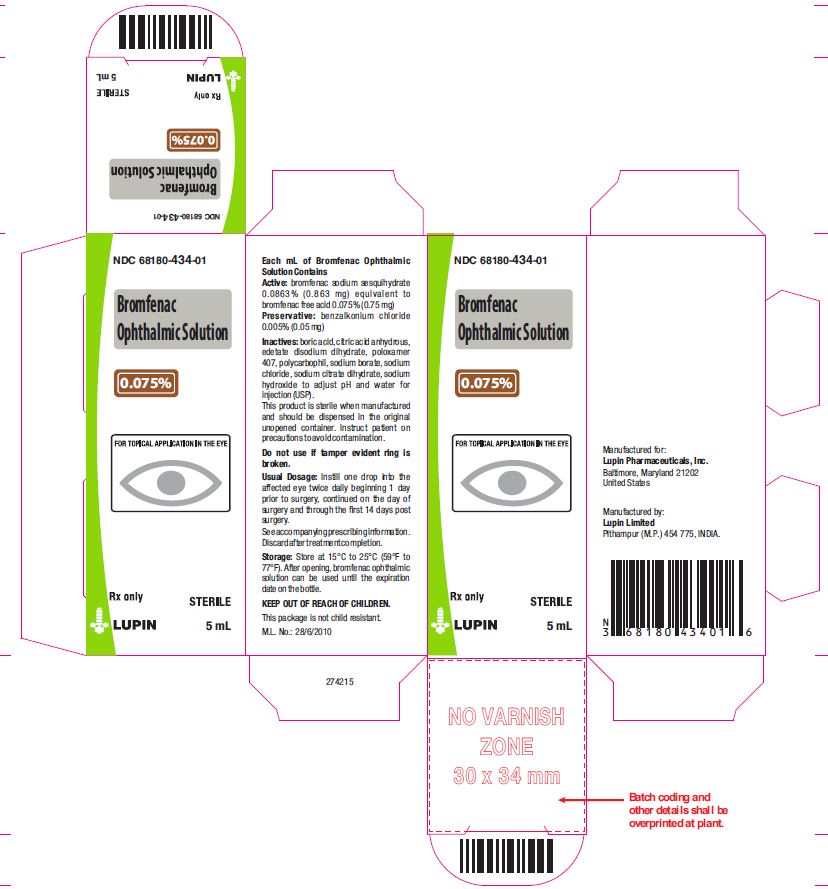
Active: Each mL contains bromfenac sodium sesquihydrate 0.0863% (0.863 mg) equivalent to bromfenac free acid 0.075% (0.75 mg)
Preservative: benzalkonium chloride 0.005% (0.05 mg)
Inactives: boric acid, citric acid anhydrous, edetate disodium dihydrate, poloxamer 407, polycarbophil, sodium borate, sodium chloride, sodium citrate dihydrate, sodium hydroxide to adjust pH and water for injection (USP).
12. Bromfenac Ophthlamic Solution - Clinical Pharmacology
12.1 Mechanism of Action
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) that has anti-inflammatory activity. The mechanism of its action is thought to be due to its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed in animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term carcinogenicity studies in rats and mice given oral doses of bromfenac up to 0.6 mg/kg/day (129 times a unilateral daily dose assuming 100% absorbed, on a mg/m2 basis) and 5 mg/kg/day (540 times a unilateral daily dose on a mg/m2 basis), respectively revealed no significant increases in tumor incidence.
Bromfenac did not show mutagenic potential in various mutagenicity studies, including the bacterial reverse mutation, chromosomal aberration, and micronucleus tests.
Bromfenac did not impair fertility when administered orally to male and female rats at doses up to 0.9 mg/kg/day and 0.3 mg/kg/day, respectively (195 and 65 times a unilateral daily dose, respectively, on a mg/m2 basis).
14. Clinical Studies
14.1 Ocular Inflammation and Pain
Clinical efficacy was evaluated in 2 multi-centered, randomized, double-masked, parallel group, placebo-controlled US trials in which subjects requiring cataract surgery were assigned to receive bromfenac ophthalmic solution or vehicle. Patients undergoing cataract surgery self- administered bromfenac ophthalmic solution or vehicle twice daily, beginning 1 day prior to surgery, continuing the day of surgery and for 14 days after surgery. Clearance of ocular inflammation was assessed on Days 1, 8, 15, and 29 using slit lamp biomicroscopy. The primary efficacy endpoint was the proportion of subjects with anterior chamber cell (ACC) grade 0 at Day 15. The secondary efficacy endpoint was the proportion of subjects who were pain free after cataract surgery as assessed using a Visual Analog Scale.
| Proportion of Subjects with Cleared Ocular Inflammation, ACC Grade 0 |
||||
| Visit | Bromfenac ophthalmic solution | Vehicle | Treatment Difference (95% CI) |
|
| Study 1 | Day 8 | 54/168 (32.1%) | 7/85 (8.2%) | 23.9% (14.7%, 33.1%) |
| Day 15 | 96/168 (57.1%) | 16/85 (18.8%) | 38.3% (27.1%, 49.5%) |
|
| Study 2 | Day 8 | 40/168 (23.8%) | 8/85 (9.4%) | 14.4% (5.5%, 23.3%) |
| Day 15 | 64/168 (38.1%) | 19/85 (22.4%) | 15.7% (4.2%, 27.3%) |
|
| Proportion of Subjects who were Pain Free |
||||
| Study 1 | Day 1 | 129/168 (76.8%) | 41/85 (48.2%) | 28.6% (16.2%, 40.9%) |
| Study 2 | Day 1 | 138/168 (82.1%) | 53/85 (62.4%) | 19.8% (8.0%, 31.6%) |
16. How is Bromfenac Ophthlamic Solution supplied
Bromfenac ophthalmic solution, 0.075% is supplied in white opaque low density polyethylene (LDPE) bottle closed with natural LDPE Nozzle and sealed with gray colored HDPE Cap. Tamper evidence provided by tamper evident ring. Each bottle is packed in a carton.
5 mL in a 10 mL bottle
(NDC No. 68180-434-01)
Storage: Store at 15ºC to 25ºC (59ºF to 77 ºF). After opening, bromfenac ophthalmic solution can be used until the expiration date on the bottle. Discard after treatment completion.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Slow or Delayed Healing
Advise patients of the possibility that slow or delayed healing may occur while using NSAIDs.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, advise patients to administer bromfenac ophthalmic solution at least 5 minutes after instillation of other topical medications.
Concomitant Use of Contact Lenses
Advise patients not to wear contact lenses during administration of bromfenac ophthalmic solution. The preservative in this product, benzalkonium chloride, may be absorbed by soft contact lenses.
Sterility of Dropper Tip/Product Use
Advise patients to replace the bottle cap after use and do not touch the dropper tip to any surface as this may contaminate the contents.
Advise patients to thoroughly wash hands prior to using bromfenac ophthalmic solution.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Pithampur (M.P.) - 454 775
India
Revised: August 2023
Bromfenac (brome' fen ak ) Ophthalmic Solution
0.075%
Read this Instructions for Use before you start using bromfenac ophthalmic solution and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Information about bromfenac ophthalmic solution:
- Do not let the bromfenac ophthalmic solution nozzle touch your eye, fingers, or any other surfaces.
- If you are using bromfenac ophthalmic solution with other eye (ophthalmic) medicines, you should wait at least 5 minutes after using the other medicine to give your bromfenac ophthalmic solution dose.
- You should not wear contact lenses while using bromfenac ophthalmic solution.
- Put the gray cap back on the bromfenac ophthalmic solution after each use.
Before you use bromfenac ophthalmic solution for the first time:
- Break the tamper evident ring by turning cap in counterclockwise direction (SeeFIGURE A).
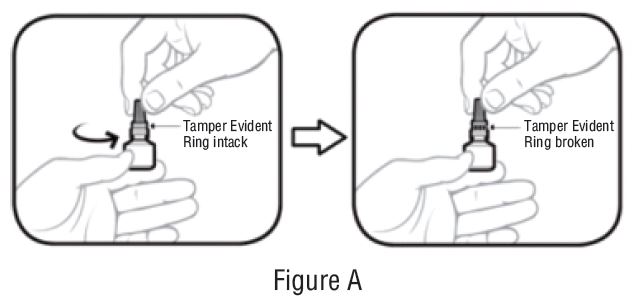
- Hold the bottle upright. Remove the gray cap by turning it in the counterclockwise direction (SeeFIGURE B).
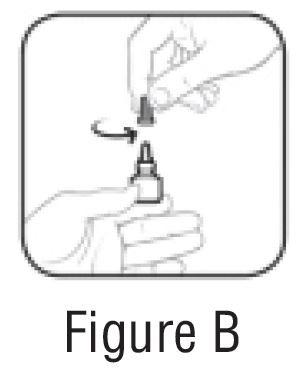
- Replace the gray cap on the bottle and close tightly (SeeFIGURE C).
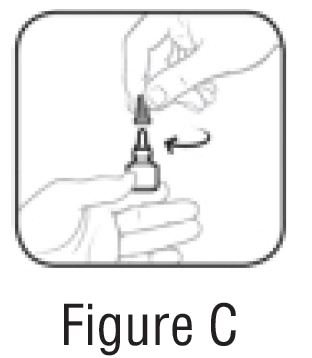
Follow Steps 1 to 5 each time you use bromfenac ophthalmic solution.
Step 1. Wash your hands well.
Step 2. Turn the closed bottle upside down (See FIGURE D).
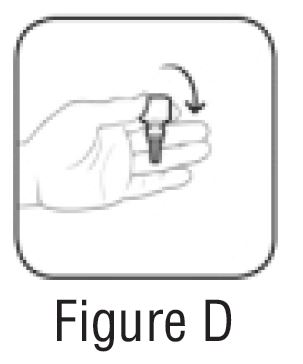
Step 3. Flick bottle firmly 1 time before each use to move the medicine into the tip of the bottle (See FIGURE E).
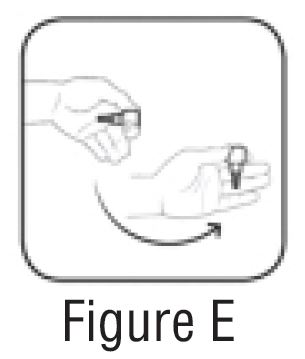
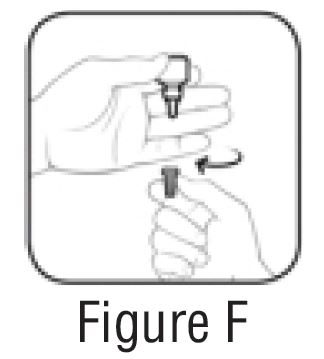
Step 4. Keep the bottle upside down and remove the gray cap by turning it in clockwise direction (See FIGURE F).
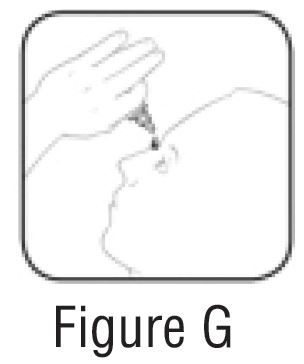
Step 5. Tilt your head back. Gently squeeze the bottle to place 1 drop into the affected eye (See FIGURE G). Replace the gray cap on the bottle and close tightly.
How do I store bromfenac ophthalmic solution?
- Store bromfenac ophthalmic solution at 15°C to 25°C (59°F to 77°F). After opening, bromfenac ophthalmic solution can be used until the expiration date on the bottle.
- Throw away the bromfenac ophthalmic solution bottle after your treatment is finished.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Pithampur (M.P.) - 454 775
India
Revised: August 2023
| BROMFENAC
bromfenac solution/ drops |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Lupin Pharmaceuticals, Inc. (089153071) |
| Registrant - LUPIN LIMITED (675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LUPIN LIMITED | 863645527 | MANUFACTURE(68180-434) , PACK(68180-434) | |
More about bromfenac ophthalmic
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (13)
- Side effects
- Dosage information
- During pregnancy
- Drug class: ophthalmic anti-inflammatory agents
- Breastfeeding
- En español