Avage Prescribing Information
Package insert / product label
Generic name: tazarotene
Dosage form: cream
Medically reviewed by Drugs.com. Last updated on Mar 8, 2024.
On This Page
- Indications and Usage
- Dosage and Administration
- Dosage Forms and Strengths
- Contraindications
- Warnings and Precautions
- Adverse Reactions/Side Effects
- Drug Interactions
- Use In Specific Populations
- Overdosage
- Description
- Clinical Pharmacology
- Nonclinical Toxicology
- Clinical Studies
- How Supplied/Storage and Handling
- Storage and Handling
- Patient Counseling Information
Highlights of Prescribing Information
AVAGE ® (tazarotene) cream, 0.1%, for topical use
Initial U.S. Approval: 1997
Indications and Usage for Avage
AVAGE® Cream, 0.1% is a retinoid indicated as an adjunctive agent for use in the mitigation (palliation) of facial fine wrinkling, facial mottled hyper- and hypopigmentation, and benign facial lentigines in patients who use comprehensive skin care and sunlight avoidance programs. (1)
Limitations of Use:
Avage Dosage and Administration
Dosage Forms and Strengths
Cream, 0.1%. (3)
Warnings and Precautions
- Embryo-Fetal Toxicity: May cause fetal harm when administered to a pregnant woman. Obtain a pregnancy test in females of reproductive potential within 2 weeks prior to initiating treatment. Advise females of reproductive potential to use effective contraception. (5.1)
- Local Irritation: Some individuals may experience excessive pruritus, burning, skin redness, or peeling. If these adverse reactions occur, discontinue AVAGE Cream until the integrity of the skin has been restored or reduce dosing interval. Avoid using AVAGE Cream on eczematous skin, as such use may cause severe irritation. (5.2)
- Photosensitivity and Risk of Sunburn: Avoid exposure to sunlight, sunlamps, and weather extremes. Wear sunscreen daily. Avoid using AVAGE Cream if the patient is also taking drugs known to be photosensitizers. (5.3)
- Lentigo Maligna: Carefully assess facial pigmented lesions of concern before application of AVAGE Cream. (5.4)
Adverse Reactions/Side Effects
Most common adverse events (occurring in ≥10% of patients) are desquamation, erythema, burning sensation, dry skin, skin irritation, and pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2017
Related/similar drugs
Botox, onabotulinumtoxinA, Xeomin, tazarotene topical, Dysport
Full Prescribing Information
1. Indications and Usage for Avage
AVAGE (tazarotene) Cream, 0.1% is indicated as an adjunctive agent for use in the mitigation (palliation) of facial fine wrinkling, facial mottled hyper- and hypopigmentation, and benign facial lentigines in patients who use comprehensive skin care and sunlight avoidance programs.
Limitations of Use:
- AVAGE Cream does not eliminate or prevent wrinkles or restore more youthful skin.
- AVAGE Cream does not reverse photoaging or repair sun damaged skin; AVAGE Cream does not mitigate coarse or deep wrinkling, tactile roughness, telangiectasia, skin laxity, keratinocytic atypia, melanocytic atypia, or dermal elastosis.
- The safety and the effectiveness of AVAGE Cream for the prevention or treatment of actinic keratoses, skin neoplasms, or lentigo maligna have not been established.
2. Avage Dosage and Administration
2.1 Assessment Prior to Treatment Initiation
Obtain a pregnancy test within 2 weeks prior to AVAGE Cream therapy. Initiate AVAGE Cream therapy during a menstrual period [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3)].
Carefully assess facial pigmented lesions of concern by a qualified physician (e.g., dermatologist) before application of AVAGE Cream [see Warnings and Precautions (5.4)].
2.2 Important Administration Instructions
Avoid accidental transfer of AVAGE Cream into eyes, mouth, or other mucous membranes. If contact with mucous membranes occurs, rinse thoroughly with water [see Warnings and Precaution (5.2)].
Wash hands thoroughly after application.
Emollients or moisturizers can be applied either before or after applying AVAGE Cream. However, ensure that the first cream or lotion has absorbed into the skin and has dried completely before subsequent cream or lotion application. Use facial moisturizers as frequently as desired [see Warnings and Precaution (5.2)].
AVAGE Cream is for topical use only. AVAGE Cream is not for ophthalmic, oral, or intravaginal use.
Use effective sunscreens and wear protective clothing while using AVAGE Cream [see Warnings and Precaution (5.3)].
2.3 Dosage and Administration Instructions
Remove any makeup before applying AVAGE Cream to the face. Dry the skin before applying the cream after face washing, bathing, or showering.
Apply a pea-sized amount once a day at bedtime to lightly cover the entire face, including the eyelids, if desired.
Wash hands thoroughly after application.
3. Dosage Forms and Strengths
Cream: 1 mg of tazarotene per gram (0.1%) of white cream in 30 gram tubes.
5. Warnings and Precautions
5.1 Embryofetal Toxicity
Based on data from animal reproduction studies, retinoid pharmacology and the potential for systemic absorption, AVAGE Cream may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from AVAGE Cream use during pregnancy; therefore, discontinue AVAGE Cream as soon as pregnancy is recognized. Tazarotene elicits malformations and developmental effects associated with retinoids after topical and oral administration to pregnant rats and rabbits during organogenesis. However, limited case reports of pregnancy in females enrolled in clinical trials for AVAGE Cream have not reported a clear association with tazarotene and major birth defects or miscarriage risk [see Contraindications (4), Use in Specific Populations (8.1)].
Systemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in these orally treated animals. Although there may be less systemic exposure in the treatment of the face alone due to less surface area for application, tazarotene is a teratogenic substance in animals, and it is not known what level of exposure is required for teratogenicity in humans [see Clinical Pharmacology (12.3)].
Advise pregnant females of the potential risk to a fetus. Obtain a pregnancy test within 2 weeks prior to AVAGE Cream therapy. Initiate AVAGE Cream therapy during a menstrual period. Advise females of reproductive potential to use effective contraception during treatment with AVAGE Cream [see Dosage and Administration (2), Use in Specific Populations (8.3)].
5.2 Local Irritation and Hypersensitivity Reactions
Local tolerability reactions (including blistering and skin desquamation) and hypersensitivity adverse reactions (including urticaria) have been observed with topical tazarotene. Application of AVAGE Cream may cause excessive irritation in the skin of certain sensitive individuals. Some individuals may experience excessive pruritus, burning, skin redness, or peeling. If these adverse reactions occur, discontinue the medication until the integrity of the skin is restored, or reduce the dosing to an interval the patient can tolerate. Closely monitor the frequency of application by carefully observing the therapeutic response and skin tolerance.
Avoid concomitant use of topical medications and cosmetics that have a strong drying effect. It is also advisable to “rest” a patient's skin until the effects of such preparations subside before use of AVAGE Cream is begun.
Avoid using AVAGE Cream on eczematous skin because such use may cause severe irritation.
Weather extremes, such as wind or cold, may be more irritating to patients using AVAGE Cream.
5.3 Photosensitivity and Risk of Sunburn
Because of heightened burning susceptibility, minimize exposure to ultraviolet rays (including sunlight and sun lamps) during the use of AVAGE Cream. Patients must be warned to use sunscreens and protective clothing when using AVAGE Cream. Advise patients with sunburn not to use AVAGE Cream until the sunburn is fully recovered.
Patients who may have considerable sun exposure because of their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using AVAGE Cream.
Avoid using AVAGE Cream if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Embryofetal toxicity [see Warnings and Precautions (5.1)]
- Photosensitivity and Risk of Sunburn [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most frequent adverse reactions reported with AVAGE Cream, 0.1% that occurred in greater than 10% of subjects, included desquamation, erythema, burning sensation, and dry skin (in descending order). Reactions that occurred in 1 to 10% of subjects, included skin irritation, pruritus, irritant contact dermatitis, stinging, rash, and cheilitis (in descending order). Common adverse events that occurred at a rate of at least 1% and at a higher rate in the AVAGE Cream group than in the vehicle group in the clinical trials are presented in the following table.
| TABLE OF ADVERSE EVENTS SEEN IN 24-WEEK CLINICAL TRIALS WITH AVAGE CREAM 0.1% | ||
| Adverse Event | AVAGE
N=567 | Vehicle
N=564 |
| Desquamation | 40% | 3% |
| Erythema | 34% | 3% |
| Burning Sensation | 26% | <1% |
| Dry Skin | 16% | 3% |
| Irritation Skin | 10% | 1% |
| Pruritus | 10% | 1% |
| Irritant Contact Dermatitis | 8% | 1% |
| Stinging | 3% | <1% |
| Rash | 3% | 1% |
| Cheilitis | 1% | 0% |
A few subjects reported adverse events at Week 0; however, for patients who were treated with AVAGE Cream, the highest number of new reports for each adverse event was at Week 2.
When combining data from the two trials, 5.3% of subjects in the AVAGE Cream group and 0.9% of subjects in the vehicle group discontinued because of adverse events.
Overall, 20/567 (3.5%) subjects in the AVAGE Cream group and 16/564 (2.8%) subjects in the vehicle group reported adverse events (including edema, irritation, and inflammation) directly related to the eye or eyelid. The majority of these conditions were mild.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of tazarotene. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: blister, dermatitis, urticaria, skin exfoliation, skin discoloration (including skin hyperpigmentation or skin hypopigmentation), swelling at or near application sites, and pain.
7. Drug Interactions
No formal drug-drug interaction studies were conducted with AVAGE Cream.
In a trial of 27 healthy female subjects between the ages of 20-55 years receiving a combination oral contraceptive tablet containing 1 mg norethindrone and 35 mcg ethinyl estradiol, concomitant use of tazarotene administered as 1.1 mg orally (mean ± SD Cmax and AUC0-24 of tazarotenic acid were 28.9 ± 9.4 ng/mL and 120.6 ± 28.5 ng*h/mL) did not affect the pharmacokinetics of norethindrone and ethinyl estradiol over a complete cycle.
The impact of tazarotene on the pharmacokinetics of progestin only oral contraceptives (i.e., minipills) has not been evaluated.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, AVAGE Cream may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from AVAGE Cream during pregnancy; therefore, AVAGE Cream should be discontinued as soon as pregnancy is recognized [see Contraindications (4), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]. Limited case reports of pregnancy in females enrolled in clinical trials for AVAGE Cream have not established a clear association with tazarotene and major birth defects or miscarriage risk. Because the exact timing and extent of exposure in relation to the gestational age are not certain, the significance of these findings is unknown.
In animal reproduction studies with pregnant rats, tazarotene dosed topically during organogenesis at 2 times the maximum systemic exposure in subjects treated with the maximum recommended human dose (MRHD) of tazarotene cream, 0.1% resulted in reduced fetal body weights and reduced skeletal ossification. In animal reproduction studies with pregnant rabbits dosed topically with tazarotene gel at 26 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%, there was a single incident of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.
In animal reproduction studies with pregnant rats and rabbits, tazarotene dosed orally during organogenesis at 2 and 52 times, respectively, the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1% resulted in malformations, fetal toxicity, developmental delays, and/or behavioral delays. In pregnant rats, tazarotene dosed orally prior to mating through early gestation resulted in decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations at doses approximately 7 times higher than the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1% [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In rats, a tazarotene 0.05% gel formulation dosed topically during gestation days 6 through 17 at 0.25 mg/kg/day, which represented 2 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1% (i.e., 2 mg/cm2 over a 15% body surface area), resulted in reduced fetal body weights and reduced skeletal ossification. Rabbits dosed topically with 0.25 mg/kg/day tazarotene gel, which represented 26 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%, during gestation days 6 through 18, had a single incident of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.
When tazarotene was given orally to animals, developmental delays were seen in rats, and malformations and post-implantation loss were observed in rats and rabbits at doses representing 2 and 52 times, respectively, the maximum systemic exposure seen in subjects treated with the MRHD of tazarotene cream, 0.1%.
In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, which represented 7 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%, classic developmental effects of retinoids were observed including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights. A low incidence of retinoid-related malformations was observed at that dose.
In a pre- and postnatal development toxicity study, topical administration of tazarotene gel (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival, but did not affect the reproductive capacity of the offspring. Based on data from another study, the maximum systemic exposure in the rat would be equivalent to the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%.
8.2 Lactation
Risk Summary
There is no information regarding the presence of tazarotene in human milk, the effects on the breastfed infant, or the effects on milk production. After single topical doses of 14C-tazarotene gel to the skin of lactating rats, radioactivity was detected in rat milk. The lack of clinical data during lactation precludes a clear determination of the risk of AVAGE Cream to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AVAGE Cream and any potential adverse effects on the breastfed child from AVAGE Cream or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy testing is recommended for females of reproductive potential within 2 weeks prior to initiating AVAGE Cream therapy which should begin during a menstrual period.
Contraception
Females
Based on animal studies, AVAGE Cream may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with AVAGE Cream.
8.4 Pediatric Use
The safety and efficacy of AVAGE Cream have not been established in patients under the age of 17 years with facial fine wrinkling, facial mottled hyper- and hypopigmentation, and benign facial lentigines.
8.5 Geriatric Use
In the studies of facial fine wrinkling, facial mottled hyper- and hypopigmentation, and benign facial lentigines, 44 male subjects and 180 female subjects out of the total population of 1131 subjects were older than 65 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
10. Overdosage
AVAGE Cream is not for oral use. Oral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of Vitamin A (hypervitaminosis A) or other retinoids. . If oral ingestion occurs, monitor the patient closely and administer appropriate supportive measures, as necessary.
11. Avage Description
AVAGE Cream, 0.1% is for topical use and contains the active ingredient, tazarotene. Each gram of AVAGE Cream, 0.1% contains 1 mg of tazarotene in a white cream base.
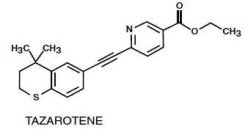
Tazarotene is a member of the acetylenic class of retinoids. Chemically, tazarotene is
ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl] nicotinate. The compound has an empirical formula of C21H21NO2S and molecular weight of 351.46. The structural formula is shown below:
AVAGE Cream contains the following inactive ingredients: benzyl alcohol 1%, carbomer homopolymer type B; carbomer 1342, edetate disodium, medium chain triglycerides, mineral oil, purified water, sodium thiosulfate, sorbitan monooleate, and sodium hydroxide to adjust pH.
12. Avage - Clinical Pharmacology
12.1 Mechanism of Action
Tazarotene is a retinoid prodrug which is converted to its active form, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three members of the retinoic acid receptor (RAR) family: RARα, RARβ, and RARγ, but shows relative selectivity for RARβ and RARγ, and may modify gene expression. The clinical significance of these findings for the mitigation of facial fine wrinkling, facial mottled hyper-and hypopigmentation, and benign facial lentigines is unknown.
12.3 Pharmacokinetics
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. Little parent compound could be detected in the plasma. Tazarotenic acid was highly bound to plasma proteins (greater than 99%). Tazarotene and tazarotenic acid were metabolized to sulfoxides, sulfones, and other polar metabolites which were eliminated through urinary and fecal pathways. The half-life of tazarotenic acid was approximately 18 hours.
Tazarotene cream 0.1% was topically applied once daily over four weeks to either the face (6 females and 2 males) or to 15% of body surface area (8 females and 8 males) in subjects with fine wrinkling and mottled hyperpigmentation. In the “face-only” dosing group, the maximum average Cmax and AUC0-24hr values of tazarotenic acid occurred on Day 15 with mean ± SD values of Cmax and AUC0-24hr of tazarotenic acid being 0.236 ± 0.255 ng/mL (N=8) and 2.44 ± 1.38 ng·hr/mL (N=8), respectively. The mean Cmax and AUC0-24hr values of tazarotenic acid from subjects in the 15% body surface area dosing group were approximately 10 times higher than those from subjects in the face-only dosing group. The single highest Cmax throughout the trial period was 3.43 ng/mL on day 29 from subjects in the 15% body surface area dosing group. Gender had no influence on the systemic bioavailability of tazarotenic acid.
Blood samples were collected from one of the two phase 3 trials to evaluate the systemic exposure following application of tazarotene cream 0.1% once daily for 24 weeks (double-blind period) followed by 28 weeks (open-label) under clinical conditions. The mean plasma tazarotenic acid concentrations, following topical treatment with tazarotene cream 0.1% over 52 weeks, ranged between 0.092 ± 0.073 ng/mL and 0.127 ± 0.142 ng/mL. The single highest observed tazarotenic acid concentration throughout the 52-week trial was 0.705 ng/mL (observed at week 36). Systemic availability of tazarotenic acid was minimal and remained steady following once daily application of tazarotene cream 0.1% to the faces of subjects in the trial for up to 52 weeks.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A long term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. Based on pharmacokinetic data from a shorter term study in rats, the highest dose of 0.125 mg/kg/day was anticipated to give systemic exposure in the rat equivalent to the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream 0.1%.
A long-term topical application study of up to 0.1% tazarotene in a gel formulation in mice, terminated at 88 weeks, showed that dose levels of 0.05, 0.125, 0.25, and 1 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals. Systemic exposure at the highest dose represented 8 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream 0.1%.
Tazarotene was non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in a human lymphocyte assay. Tazarotene was also non-mutagenic in the CHO/HGPRT mammalian cell forward gene mutation assay and was non-clastogenic in the in vivo mouse micronucleus test.
No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of tazarotene gel up to 0.125 mg/kg/day. Based on data from another study, the systemic drug exposure in the rat would be equivalent to the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream 0.1%.
No impairment of mating performance or fertility was observed in male rats treated for 70 days prior to mating with oral doses of up to 1 mg/kg/day tazarotene, which represented 4 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream 0.1%.
No effect on parameters of mating performance or fertility was observed in female rats treated for 15 days prior to mating and continuing through day 7 of gestation with oral doses of tazarotene up to 2 mg/kg/day. However, there was a significant decrease in the number of estrous stages and an increase in developmental effects at that dose, which represented 7 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream 0.1% [see Use in Specific Populations (8.1)].
14. Clinical Studies
Two double-blind, randomized vehicle-controlled trials (Trial 1 and Trial 2) enrolled 1131 subjects with mild to severe fine wrinkling, facial mottled hyper- and hypo-pigmentation, and benign facial lentigines because of sun overexposure. Both trials compared the application of AVAGE cream 0.1% to its vehicle once daily for 24 weeks to the facial skin. Treatment was as an adjunct to a comprehensive skin care and sun avoidance program that included use of sunscreens, protective clothing, and non-prescription emollient cream.
In both trials, the endpoint was the proportion of subjects achieving an improvement of at least one grade from baseline in fine wrinkling, mottled hypo- and hyper-pigmentation, and benign facial lentigines. At two to four week intervals, the severity of fine wrinkling, mottled hyper- and hypo-pigmentation, and benign facial lentigines were graded on a using a 5-point photonumeric scale (0 = none, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe).
Of 1131 subjects, approximately 97% of subjects in clinical trials were white (Caucasian) with 80% of subjects in the clinical studies having Fitzpatrick skin type classifications I-III. The distribution of subject skin types were: Type I –12%; Type II – 26%; Type III – 40%; and Type IV 22%. Subjects with skin types V and VI were not studied. Insufficient number of non-white subjects (Asian, Hispanic, or other) were studied to make an adequate determination of efficacy of AVAGE Cream in such subjects.
| Trial 1 | Trial 2 | |||
| AVAGE Cream, 0.1% N=283 | Vehicle N=280 | AVAGE Cream, 0.1% N=284 | Vehicle N=284 |
|
| 2 or more Grades Improvement | 5% | 1% | 13% | 5% |
| 1 Grade Improvement | 35% | 15% | 45% | 18% |
| No Change | 59% | 83% | 42% | 76% |
| Worsened | 1% | 1% | 0% | 1% |
Fine Wrinkling was graded on a 5-point scale (0=none, 1=minimal, 2=mild, 3=moderate, 4=severe) using a photonumeric guideline for investigators.
| Trial 1 | Trial 2 | ||||
| AVAGE Cream, 0.1% N=283 | Vehicle N=280 | AVAGE Cream, 0.1% N=284 | Vehicle N=284 |
||
| 2 or more Grades Improvement | 17% | 1% | 28% | 10% | |
| 1 Grade Improvement | 42% | 17% | 54% | 30% | |
| No Change | 41% | 80% | 18% | 59% | |
| Worsened | <1% | 3% | <1% | 1% | |
Mottled hyperpigmentation was graded on a 5-point scale (0=none, 1=minimal, 2=mild, 3=moderate, 4=severe) using a photonumeric guideline for investigators.
In the 24 week trials, efficacy was also demonstrated in mottled hypopigmentation and benign facial lentigines, which were secondary endpoints in those trials.
The duration of the mitigating effects on facial fine wrinkling, mottled hyper- and hypopigmentation, and benign facial lentigines following discontinuation of AVAGE Cream has not been studied.
16. How is Avage supplied
AVAGE (tazarotene) Cream 0.1%, containing 1 mg of tazarotene per gram of white cream is available in a 30 gram collapsible aluminum tube with a tamper-evident aluminum membrane over the opening and a white polypropylene screw cap (NDC 0023-9236-30).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryofetal Toxicity
Inform females of reproductive potential of the potential risk to a fetus. Advise these patients to use effective contraception during treatment with AVAGE Cream. Advise patients to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
Photosensitivity and Risk of Sunburn
Advise patients to avoid excessive sun exposure and to use of sunscreens and protective measures (hat, visor). Advise patients to avoid using AVAGE if also taking other medicines may increase sensitivity to sunlight.
Important Administration Instructions
Advise patients of the following:
- Use AVAGE Cream on the face once per day, at bedtime.
- AVAGE Cream is for topical use only. Do not apply to eyes, mouth, or other mucous membranes. The cream may cause severe redness, itching, burning, stinging, and peeling. Avoid accidental transfer of AVAGE Cream into eyes, mouth, or other mucous membranes. If contact with mucous membranes occurs, rinse thoroughly with water. Seek medical attention if eye irritation continues. Wash hands thoroughly after applying AVAGE Cream.
- Gently wash face with a mild soap before applying the cream.
- Dry skin before applying the cream.
- Apply only a small pea sized amount (about 1/4 inch or 5 millimeter diameter) to lightly cover the entire face.
- Apply emollients or moisturizers before or after tazarotene cream and ensure that the first cream or lotion has absorbed into the skin and dried completely.
- In the morning, apply a moisturizing sunscreen.
© 2017 Allergan. All rights reserved.
All trademarks are the property of their respective owners.
Irvine, CA 92612
Made in the U.S.A.

|
This Patient Information has been approved by the U.S. Food and Drug Administration |
Revised: July/2017 |
|
| PATIENT INFORMATION
AVAGE (ah-vaj) (tazarotene) Cream, 0.1% |
||
| Important information: AVAGE Cream is for use on skin only. Do not use AVAGE Cream in your eyes, mouth, or vagina. | ||
|
What is the most important information I should know about AVAGE Cream?
|
||
| What is AVAGE Cream?
AVAGE Cream is a prescription medicine used on the skin (topical) that may reduce fine facial wrinkles and certain types of dark and light spots on the face in people who use a total skin care program and avoid sunlight.
|
||
| Who should not use AVAGE Cream?
Do not use AVAGE Cream if you:
|
||
| What should I tell my doctor before using AVAGE Cream?
Before you use AVAGE Cream, tell your doctor about all of your medical conditions, including if you:
Certain medicines, vitamins, or supplements may make your skin more sensitive to sunlight. Also, tell your doctor about any cosmetics you use, including moisturizers, creams, lotions, or products that can dry out your skin. |
||
How should I use AVAGE Cream?
|
||
Follow these instructions for applying AVAGE Cream:
|
||
What should I avoid while using AVAGE Cream?
|
||
| What are the possible side effects of AVAGE Cream?
AVAGE Cream may cause serious side effects, including:
|
||
| The most common side effects of AVAGE Cream include peeling, redness, burning, dry or irritated skin, and itching. These are not all the side effects possible of AVAGE Cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store AVAGE Cream?
|
||
| General information about the safe and effective use of AVAGE Cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AVAGE Cream for a condition for which it was not prescribed. Do not give AVAGE Cream to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about AVAGE Cream that is written for health professionals. |
||
| What are the ingredients of AVAGE Cream?
Active ingredient: tazarotene Inactive ingredients: benzyl alcohol, carbomer homopolymer type B, carbomer 1342, edetate disodium, medium chain triglycerides, mineral oil, purified water, sodium thiosulfate, sorbitan monooleate and sodium hydroxide to adjust pH Manufactured by: Allergan Sales, LLC., Waco, Texas |
||
| © 2017 Allergan. All rights reserved. All trademarks are the property of their respective owners. Irvine, CA 92612 Made in the U.S.A. For more information call 1-800-678-1605. |
 |
|
73291US11
| AVAGE
tazarotene cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |
Frequently asked questions
- What are the different brands of tazarotene?
- Is Tazorac better than Retin-A?
- What does it do to your skin?
- How long should you use Duobrii?
- What happens if you use too much Duobrii?
- Can Tazorac and Differin be used together?
More about Avage (tazarotene topical)
- Check interactions
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Breastfeeding